Nicolas Potier, Benjamin Touzé, Jean-Marc Lo-Guidice, Sébastien Anthérieu, Guillaume Grzych*
Nitrous oxide (N2O) is a gas used in medicine for its anesthetic and analgesic properties, and in industry as an oxidizing gas or propellant. This gas is also available for self-service food use. In recent years, its use has been increasingly diverted to recreational purposes. Between 2020 and 2021, the number of serious cases of poisoning rose by 320% in France, demonstrating a major public health concern. It is also a problem for road safety, with an increase in the number of road accidents linked to the consumption of this gas. Nitrous oxide causes short-term effects (euphoria, disorientation, etc.) and long-term effects (sensory and motor neurological disorders, thrombosis). This gas acts on the central nervous system, inducing anxiolytic, anesthetic, antidepressant and analgesic effects. Nitrous oxide is difficult to quantify due to its rapid elimination. Biological investigation thus relies on secondary anomalies linked to vitamin B12 inactivation: increased levels of methylmalonic acid and homocysteine. However, these two biological markers are not specific to nitrous oxide intoxication. To improve patient management, we need to find new biological markers that are more specific to nitrous oxide poisoning.
Nitrous oxide - History, uses and physicochemical properties
Nitrous oxide (N2O) was first discovered in 1772 by the chemist Joseph Priestley.
In the 19th century, it was used recreationally at British funfairs for its euphoric effect. Its analgesic action was accidentally observed by Horace Wells in 1844, during a show. Wells used the gas to pull out a molar, confirming its anesthetic action. He later used nitrous oxide on his patients. In 1845, Horace Wells attempted to demonstrate its therapeutic applicability to the medical community, but was unsuccessful.
It wasn't until 1867 that it arrived in France [1]. Today, nitrous oxide is still used as a mild analgesic under the name MEOPA (for Mixture équimolaire oxygène protoxyde d'azote). Nitrous oxide also has other uses.
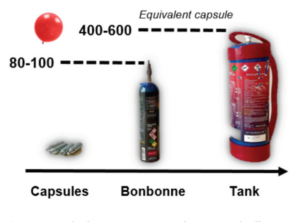
In the food industry, it is used as a foaming and mixing agent (E942). It is also used in the automotive industry, in the propulsion systems of racing cars. In the space industry, nitrous oxide can be used as a liquid oxidizer for rocket propulsion [2]. In recent years, however, its use has been increasingly diverted. Initially sold freely in the form of whipped cream siphon capsules, new containers have emerged for "recreational" use only. Capsules were transformed into carboys (capacity equivalent to 80-100 capsules) and tanks (400-600 capsules) (figure 1)making gas cheaper and simpler to use, leading to more frequent and prolonged use.
Nitrous oxide has the chemical formula N2O, and is a colorless, odorless gas with a slightly sweet taste and odor. It is denser than air and soluble in water. It is an oxidizing and oxidizing gas, giving it its propulsive properties.
Nitrous oxide is stable at room temperature [3].
Epidemiology and clinical consequences
According to data collected in 2022 by Santé publique France, around 4.3% of adults have experimented with nitrous oxide at least once in their lives. Among 18-24 year-olds, prevalence is higher, with 13.7% having used nitrous oxide at least once in their lives, and 3.2% having used it during the year. Consumers are predominantly male, with an average age of 25 [4]. The latest report from France's Agence nationale de sécurité du médicament et des produits de santé (ANSM) in 2021 shows a significant increase in the number of intoxication cases in France linked to the recreational use of nitrous oxide. The number of serious cases rose from 82 to 265 between 2020 and 2021, an increase of 320%, with daily consumption accounting for half of all cases, and cylinder consumption the preferred method (71.6% of cases).
The number of intoxication cases among minors has doubled from 16 to 37 between 2020 and 2021 [5].
These alarming increases in the number of cases of nitrous oxide poisoning represent a major public health concern. The number of road accidents linked to nitrous oxide consumption has risen sharply in recent years. Drivers who have consumed nitrous oxide have reduced reflexes and impaired motor coordination, increasing the risk of road accidents. In the Netherlands, the number of police reports on road incidents linked to the consumption of this gas rose from 60 to 960 between 2016 and 2019 [6].
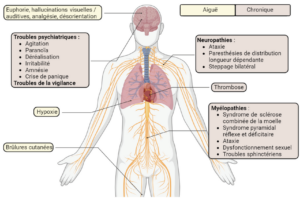
The growing popularity of nitrous oxide, and the increase in consumption levels, both in terms of quantity and frequency, have led to an increase in cases of toxicity. Indeed, nitrous oxide consumption can lead to side effects (figure 2). These effects may be due to direct or indirect exposure, but the underlying mechanisms are not fully understood. Nitrous oxide damages the peripheral and central nervous system, leading to severe paralysis in some users, often associated with myelopathy or neuropathy. Clinical neurological signs are diverse, including paresthesias, ataxias and combined spinal cord sclerosis [7]. Thrombosis has also been reported in some patients, and is linked to chronic use of this gas. Skin burns of the contact frostbite type have also been observed in connection with the mode of consumption. The cylinder is often positioned between the consumer's thighs to collect the gas in a balloon. However, when the nitrous oxide is released, the cylinder cools rapidly (-40°C) and, with the anesthetic effect of this gas, the consumer has no withdrawal reflex.
This is why users don't feel the sensation of intense cold, increasing the severity of skin burns [8].
Addiction to nitrous oxide is less and less questioned. In fact, chronic use of this substance can lead to tolerance, forcing users to take higher doses to feel the euphoric effects [9].
Fidalgo et al. have highlighted nitrous oxide use disorders according to the DSM-5 diagnosis
(Diagnostic and Statistical Manual of Mental Disorders, 5th edition) [10].
Pharmacodynamics
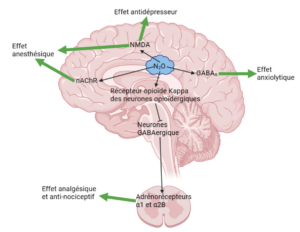
The mechanism of action of nitrous oxide is not yet fully understood. It is thought to act on receptors for glutamate, γ-aminobutyric acid (GABA for gamma-aminobutyric acid), kappa opioids, histamines, dopaminergics and α1 and α2B adrenergics, thus producing weak anesthetic, analgesic, antinociceptive, anxiolytic and antidepressant effects [13] (Figure 3).
These pharmacological effects are dependent on nitrous oxide concentration [11].
Nitrous oxide has an antagonistic effect on the N-methyl-D-aspartate (NMDA) receptor, a subtype of glutamate receptors, which is thought to be the main target responsible for the anesthetic effect.
Nitrous oxide has only a weak antagonistic effect on other glutamate receptor subtypes (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate). This anesthetic effect is also induced by inhibition of nicotinic acetylcholine receptors (nAChR).
The analgesic effect of nitrous oxide is linked to the activation of kappa opioid receptors in the periaqueductal gray matter of the midbrain. This activation induces blockade of GABAergic bridge neurons, leading to activation of α1 and α2β adrenoreceptors in the spinal cord. Activation of these receptors is responsible for reduced discharge of second-order neurons, implying reduced transmission of pain information.
Activation of GABAA receptors is involved in the anxiolytic effect of nitrous oxide. Nitrous oxide's antagonistic effect on the NMDA receptor also produces an antidepressant effect [14].
Impact on metabolism and biological markers
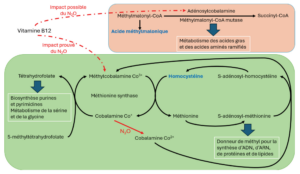
From a metabolic point of view, nitrous oxide induces oxidation of the cobalt ion Co+, present in the corrinoid nucleus of vitamin B12, to the cobalt ion Co2+, thus disrupting vitamin B12-related metabolic pathways, notably the monocarbon cycle [15,16].
Methionine synthase, a cytosolic enzyme, uses methylcobalamin-Co3+ as a cofactor for the transfer of a methyl group onto homocysteine to form methionine. This methyl transfer transforms methylcobalamin-Co3+ into cobalamin.
Co+ which is regenerated to methylcobalamine-Co3+ via the folate cycle. Cobalamin-Co+ is used in the folate cycle to convert 5-methyltetrahydrofolate into tetrahydrofolate [17].
Methionine, formed via methioninesynthase, is activated to S-adenosyl-methionine to enable the addition of methyl groups for the synthesis of DNA, RNA, proteins, lipids and myelin components. S-adenosylmethionine also enables methylation of cobalamin-Co2+ from the diet, or oxidation of cobalamin-Co+ by nitrous oxide, to methylcobalamin-Co3+ by the action of methionine synthase reductase [18].
The oxidation of cobalamin Co+ by nitrous oxide to cobalamin Co2+ prevents the regeneration of methylcobalamin-Co3+ via the folate cycle.
As a result, methionine synthase activity is reduced, leading to an accumulation of homocysteine and a depletion of the methionine pool. The folate cycle, which enables purine and pyrimidine biosynthesis and is involved in serine and glycine metabolism, is also affected [19]. In addition, methionine synthase uses S-adenosyl methionine to restore the methylcobalamin-Co3+ stock from cobalamin-Co2+, thereby amplifying methionine depletion and homocysteine accumulation [18].
Blocking this metabolic pathway with nitrous oxide leads to an accumulation of homocysteine and a decrease in methionine. This increase in homocysteine levels can be used as an indicator, albeit not a very specific one, of nitrous oxide toxicity. In fact, increased plasma homocysteine concentration is also induced by vitamin B6 or B9 deficiency, renal failure, hypothyroidism, the presence of a tumor and coffee or alcohol consumption [20].
Nitrous oxide is also thought to affect another active form of vitamin B12: adenosylcobalamin. Adenosylcobalamin is the cofactor of methylmalonyl-CoA-mutase, which converts methylmalonyl-CoA into succinyl-CoA, involved in the metabolism of fatty acids and branched-chain amino acids. Consequently, inactivating vitamin B12, and thus blocking the enzymatic activity of methylmalonyl-CoAmutase, leads to an increase in the metabolism of methylmalonyl-CoA to methylmalonic acid. Methylmalonic acid can be exploited as a non-specific indicator of nitrous oxide toxicity [20] (figure 4). The lack of specificity is consistent with the increase in plasma methylmalonic acid levels during renal failure, intestinal infection and certain metabolic diseases [21]. Furthermore, elevated methylmalonic acid is not systematically found in cases of exposure to nitrous oxide [18].
In addition, an increase in the number of nitrous oxide users taking vitamin B12 supplements has been observed [21]. This vitamin B12 supplementation is taken by users in the belief that it will reduce neurological disorders, but these disorders are functional rather than quantitative [22]. This self-medication may normalize plasma methylmalonic acid concentrations; however, high homocysteine levels may be maintained if nitrous oxide consumption is not stopped [ 2 3 ] .
The mechanisms behind nitrous oxide-induced neurological damage have yet to be determined with any certainty.
Nitrous oxide can cause demyelination and axonal damage. Demyelinating lesions generally have a more favorable prognosis due to the possibility of remyelination, whereas axonal loss is irreversible. In a study published in 2024, patients with a demyelinating profile had higher severity of clinical signs but lower hyperhomocysthemia compared with patients with axonal involvement. This study highlighted the potential role of metabolic parameters as biological markers for understanding the pathophysiology of nitrous oxide [24].
Pharmaco-toxicokinetics
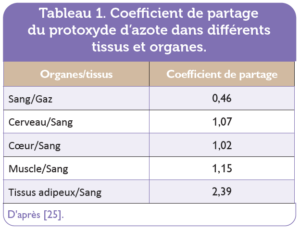
Nitrous oxide is mainly absorbed from the lungs, and is rapidly distributed throughout the body, reaching all tissues, particularly highly vascularized ones. The blood/air partition coefficient of nitrous oxide is low (0.46), and nitrous oxide concentrations between the various organs of the human body are relatively close to those in blood, leading to rapid saturation of nitrous oxide in blood and other tissues [25]. The nitrous oxide partition coefficients of certain organs and tissues/blood are shown in Table table 1. The rapid pharmacological effects of nitrous oxide, and the rapid disappearance of these effects after exposure has ceased, are due to its physicochemical properties. Nitrous oxide crosses the fetoplacental barrier.
Nitrous oxide is not metabolized by the human body. Nevertheless, a small fraction of absorbed nitrous oxide can be metabolized by reduction by bacteria present in the digestive tract [26]. Elimination is mainly via the lungs, with a minor proportion eliminated via the skin and urine. Cutaneous elimination accounts for around 6% of absorbed nitrous oxide, and varies according to skin temperature [27-29]. Complete elimination of this gas from the blood is achieved 16 hours after the end of exposure [27].
Nitrous oxide diffuses into cavities containing air (intestine, pneumocephalus, pleural cavity).
Since nitrous oxide is more widely distributed in the body than N2, inhalation of a large quantity of nitrous oxide can lead to an increase in the volume and pressure of these air-filled cavities, resulting in disorders such as meteorism or intracranial hypertension [30].
Research prospects
Currently, when treating nitrous oxide poisoning in the emergency department, a metabolic workup is carried out, including a vitamin B6, B9 (folate) and B12 assay, combined with a methylmalonic acid and homocysteine assay. A biochemical work-up is also carried out to assess the patient's renal and liver function. Today, the literature shows that homocysteine is a marker of consumption and methylmalonic acid a marker of severity [31].
However, these biological markers are not specific to nitrous oxide intoxication, and other factors can modify their concentrations, such as nutritional deficiency (vitamin B6, B9, B12), renal insufficiency, or even metabolic disease (CBS, MTHFR, MS).
There is therefore a real need to find new, more specific biological markers of nitrous oxide intoxication, which would enable better patient management and the development of new therapeutic targets.
A promising approach would be to study its metabolic impact. We now know that nitrous oxide inactivates vitamin B12, leading to a cascade of metabolic disturbances. Metabolomics studies will make it possible to study the metabolic disturbances caused by nitrous oxide in a comprehensive way. On the other hand, the study of deregulated microRNAs after nitrous oxide exposure could also be an interesting approach for identifying new biomarkers of effect or exposure. Indeed, microRNAs are relatively stable in biological fluids, and their detection at systemic level may reflect tissue damage.
These innovative approaches will make it possible to identify deregulated metabolites and/or microRNAs in the blood of nitrous oxide consumers, which could constitute relevant non-invasive biomarkers for the early detection of nitrous oxide intoxication.
These studies should pave the way for the future development of a diagnostic test for screening and monitoring patients, improving the management of nitrous oxide poisoning.
Nicolas Potier and Benjamin Touzé contributed equally to the writing of this article.
Declaration of interests: the authors declare that they have no interests.
Table 1. Partition coefficient
Scientific dossier
◗t nitrous oxide poisoning rose by 320% in France between 2020 and 2021.
◗tThis gas, used for its anesthetic, anxiolytic and antidepressant effects, is now being diverted recreationally.
◗t known sideeffects include road accidents, neurological disorders, thrombosis and skin burns.
◗t intoxicationis linked to vitamin B12 inactivation, which increases nutritional functional markers such as homocysteine and methylmalonic acid.
◗t current biologicalmarkers are not specific for nitrous oxide intoxication and cannot attest with certainty to consumption.
◗t Medical research mustbe stepped up to identify new biomarkers to improve diagnosis and follow-up.
Points to remember
[1] Goerig M, Schulte am Esch J. History of nitrous oxide-with special
reference to its early use in Germany. Best Pract Res Clin Anaesthesiol.
2001;15:313-38. -doi.org/10.1053/bean.2001.0165.
[2] Randhawa G, Bodenham A. The increasing recreational use of
nitrous oxide: history revisited. BJA Br J Anaesth. 2016;116:321-4.
doi.org/10.1093/bja/aev297.
[3] NITROUS OXIDE. CAMEO Chemicals. NOAA n.d. https://cameochemicals.
noaa.gov/chemical/8909 (accessed February 5, 2024).
[4] Lahaie E, Andler R, Beck F, Nguyen-Thanh V. Consumption levels
of CBD and nitrous oxide in the adult population of France
metropolitan France in 2022 n.a. Santé publique France. www.santepubliquefrance.
en/health-determinants/illicit-drugs/documents/studies/
consumption-levels-of-cbd-and-nitrogen-oxide-in-population-
adultes-en-france-metropolitaine-en-2022 (accessed March
21, 2024).
[5] ANSM. News - Nitrous oxide poisoning: ANSM publishes a report
a diagnostic and management aid for professionals
https://ansm.sante.fr/actualites/intoxication-auprotoxyde-
dazote-lansm-publishes-a-document-helping-diagnosis-
a-la-prise-en-charge-pour-les-professionnels-de-sante (accessed
February 1, 2024).
[6] van Hulzen D. Toename lachgas-incidenten in verkeer: ballonnetje
moet kunnen, zegt bestuurder. OUR news. 2019. https://nos.nl/
artikel/2297180-toename-lachgas-incidenten-in-verkeer-ballonnetjemoet-
kunnen-zegt-bestuurder (accessed March 28, 2024).
[7] Layzer RB. Myeloneuropathy after prolonged exposure to nitrous oxide.
Lancet. 1978;2(8102):1227-30. doi: 10.1016/s0140-6736(78)92101-3.
[8] Tillet P, Bekara F, Boissiere F et al. Burns caused by the misuse of the
nitrous oxide cylinder. Ann Chir Plast Esthét. 2023;68(3):180-3.
doi.org/10.1016/j.anplas.2023.01.005.
[9] Gillman MA. Nitrous oxide, an opioid addictive agent: Review
of the evidence. Am J Med 1986;81:97-102. doi.org/10.1016/0002-
9343(86)90189-0.
[10] Fidalgo M, Prud'homme T, Allio A et al. Nitrous oxide: What do we know?
know about its use disorder potential? Results of the French Monitoring
Center for Addiction network survey and literature review. Subst Abus.
2019;40(1):33-42. doi: 10.1080/08897077.2019.1573210.
[11] Gernez E, Lee GR, Niguet JP et al. Nitrous Oxide Abuse: Clinical
Outcomes, Pharmacology, Pharmacokinetics, Toxicity and Impact on
Metabolism. Toxics. 2023;11(12):962. doi: 10.3390/toxics11120962.
[12] Garakani A, Jaffe RJ, Savla D et al. Neurologic, psychiatric, and
other medical manifestations of nitrous oxide abuse: A systematic review
of the case literature. Am J Addict. 2016;25(5):358-69. doi: 10.1111/
ajad.12372.
[13] Chien WH, Huang MC, Chen LY. Psychiatric and Other Medical
Manifestations of Nitrous Oxide Abuse: Implications From Case Series. J Clin
Psychopharmacol. 2020;40(1):80-3. doi: 10.1097/JCP.0000000000001151.
[14] European Monitoring Centre for Drugs and Drug Addiction.
Recreational use of nitrous oxide: a growing concern for Europe 2022.
www.emcdda.europa.eu/publications/rapid-communication/recreational-
use-nitrous-oxide-growing-concern-europe_en (accessed February
15, 2024).
[15] Blackburn R, Kyaw M, John Swallow A. Reaction of Cob (I)
alamin with nitrous oxide and Cob (III) alamin. J Chem Soc Faraday
Trans 1 Phys Chem Condens Phases. 1977;73:250-5. doi.org/10.1039/
F19777300250.
[16] Ducker GS, Rabinowitz JD. One-Carbon Metabolism in Health and
Disease. Cell Metab. 2017;25(1):27-42. doi: 10.1016/j.cmet.2016.08.009.
[17] McCaddon A, Regland B, Hudson P, Davies G. Functional vitamin
B12 deficiency and Alzheimer disease. Neurology 2002;58:1395-9.
doi.org/10.1212/WNL.58.9.1395.
[18] Lucas A, Noyce AJ, Gernez E et al. Nitrous oxide abuse direct measurement
for diagnosis and follow-up: update on kinetics and impact on metabolic
pathways. Clin Chem Lab Med. 2024. doi: 10.1515/cclm-2023-1252.
[19] Sanders RD, Weimann J, Maze M. Biologic effects of nitrous oxide: a
mechanistic and toxicologic review. Anesthesiology. 2008;109(4):707-22.
doi: 10.1097/ALN.0b013e3181870a17.
[20] Blin J, Guerlais M, Masson D et al. The toxicology of protoxide
of nitrogen. Rev Franc Lab. 2021;2021:48-53. doi.org/10.1016/S1773-
035X(21)00252-5.
[21] Grzych G, Gernez E, Deheul S, Kim I. Methylmalonic acid:
a specific marker of chronic protoxide intoxication
of nitrogen? Rev Médecine Interne. 2022;43:197-8. doi.org/10.1016/j.revmed.
2022.01.001.
[22] Swart G, Blair C, Lu Z, Yogendran S et al. Nitrous oxide-induced
myeloneuropathy. Eur J Neurol. 2021;28(12):3938-44. doi: 10.1111/
ene.15077.
[23] Waclawik AJ, Luzzio CC, Juhasz-Pocsine K, Hamilton V.
Myeloneuropathy from nitrous oxide abuse: unusually high methylmalonic
acid and homocysteine levels. WMJ. 2003;102(4):43-5.
[24] Grzych G, Scuccimarra M, Plasse L et al. Understanding
Neuropathy Features in the Context of Nitrous Oxide Abuse: A Combined
Electrophysiological and Metabolic Approach n.d. Biomedicines.
2024;12(2):429. www.mdpi.com/2227-9059/12/2/429 (accessed
March 27, 2024).
[25] Kreuer S, Bruhn J, Wilhelm W, Bouillon T. Pharmakokinetische/
pharmakodynamische Modelle für Inhalationsanästhetika. Anaesthesist
2007;56:538-56. doi.org/10.1007/s00101-007-1188-7.
[26] Hong K, Trudell JR, O'Neil JR, Cohen EN. Metabolism of
Nitrous Oxide by Human and Rat Intestinal Contents. Anesthesiology.
1980;52:16-9. doi.org/10.1097/00000542-198001000-00004.
[27] Institut national de recherche et de sécurité. Nitrous oxide -
Toxicological data sheet no. 267 2018. www.inrs.fr/publications/bdd/fichetox/
fiche.html?refINRS=FICHETOX_267 (accessed February 13, 2024).
[28] Cullen, B.F., Eger, E.I. Diffusion of nitrous oxide, cyclopropane, and
halothane through human skin and amniotic membrane. Anesthesiology.
1972;36:168-73. doi.org/10.1097/00000542-197202000-00019.
[29] Stoelting RK, Eger EI 2nd. Percutaneous loss of nitrous oxide, cyclopropane,
ether and halothane in man. Anesthesiology. 1969;30(3):278-83.
doi: 10.1097/00000542-196903000-00008.
[30] Buhre W, Disma N, Hendrickx J et al. European Society of
Anaesthesiology Task Force on Nitrous Oxide: a narrative review
of its role in clinical practice. Br J Anaesth. 2019;122(5):587-604.
doi: 10.1016/j.bja.2019.01.023.
[31] Grzych G, Deheul S, Gernez E et al. Comparison of biomarker
for diagnosis of nitrous oxide abuse: challenge of cobalamin metabolic
parameters, a retrospective study. J Neurol. 2023;270(4):2237-45.
doi: 10.1007/s00415-023-11570-z.










 Make a donation
Make a donation